-
×
 Multi Fold Hand Towel Dispenser White - Fits All Interfold Xpress Towels
2 × €31.34
Multi Fold Hand Towel Dispenser White - Fits All Interfold Xpress Towels
2 × €31.34 -
×
 Slip Stop Leak collector - Hazard Sign Collection Unit
1 × €173.89
Slip Stop Leak collector - Hazard Sign Collection Unit
1 × €173.89 -
×
 Centrefeed Blue Wiper Rolls 30 Roll Deal 5Pk - Kitchen Catering Wipe
1 × €76.28
Centrefeed Blue Wiper Rolls 30 Roll Deal 5Pk - Kitchen Catering Wipe
1 × €76.28 -
×
 Henry Hoover Vacuum Cleaner Centre Selco.ie
1 × €315.86
Henry Hoover Vacuum Cleaner Centre Selco.ie
1 × €315.86 -
×
 Brown Kraft Hotdog Box Food To Go, Takeaway Food Packaging
1 × €71.34
Brown Kraft Hotdog Box Food To Go, Takeaway Food Packaging
1 × €71.34 -
×
 Back Pack Vacuum Cleaner -Battery Hoover -
1 × €1,961.85
Back Pack Vacuum Cleaner -Battery Hoover -
1 × €1,961.85 -
×
 White Centrefeed Roll 2ply 700sh 6 Pack - Pure Soft Wiping Paper
1 × €23.64
White Centrefeed Roll 2ply 700sh 6 Pack - Pure Soft Wiping Paper
1 × €23.64 -
×
 Amethyst Deodoriser For Odour Removal
1 × €26.31
Amethyst Deodoriser For Odour Removal
1 × €26.31 -
×
 Biohazard Sharps Bin Box Large Medical Bin 4 Litre Needle Box
1 × €31.89
Biohazard Sharps Bin Box Large Medical Bin 4 Litre Needle Box
1 × €31.89 -
×
 Microsafe Disinfectant Surface Wipes - Antibacterial Protection
1 × €10.74
Microsafe Disinfectant Surface Wipes - Antibacterial Protection
1 × €10.74
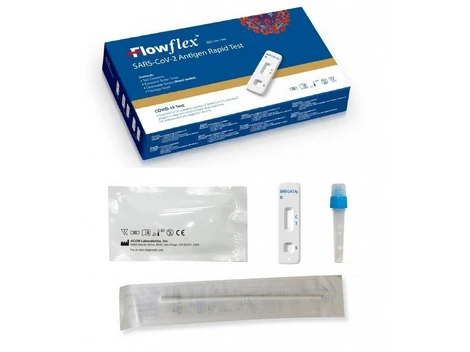
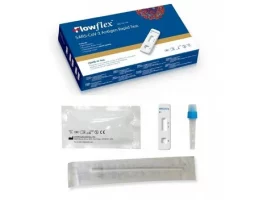
Covid 19 Nasal Rapid Testing Kits – Selco
€8.91

Categories: Best Buys, Cleaning Products, Covid, Creche and Childcare, Dentistry Hygiene Supplies, Doctor Surgery, Doctor Surgery Supplies, Facility Services, First Aid, Healthcare, Healthcare Products, Hotels Cleaning Supplies, Nursing Home Hygiene Products, Office Cleaning, Safety, School Cleaning Products
Tags: Antigen rapid saliva test kits, Antigen Rapid Test Kit, antigen test kits ireland, Back to work test kits, breaking news, Bulk Buy Covid Test Kits Ireland, buy antigen testing kits, buy covid 19 test kits, Buy Covid Test Kits Dublin, Buy Covid Test Kits In Ireland Online, Buy Covid Testing Kits In Ireland, Buy PCR test Kits Ireland, Consumable products, Covid 10 Vat Free, Covid 19 Antigen Rapid Saliva Testing Kits, Covid Kits Online Ireland, Covid Test Kit Ireland, Covid Test Kits Dublin, Covid Test Kits Ireland, Covid Test Kits Limerick, Covid Test Kits Online, Covid Test Kits Online Cork, Covid Test Online, Free Antigen Test in Ireland, Free Antigen Test Kits Ireland, Get Free Test Ireland, irish news, No vat, office antigen test kits, PCR Test Kit, sars test kit, Staff Test Kits Ireland, Vat free, wholesale antigen test kits, wholesale covid 19 test kits
-

covid-19-antigen-rapidtest-kit-Selco.ie- Free Antigen Test Kits when you buy 5 packs of face masks get 2 Test kits Free
- Antigen test using a saliva sample, so-called spit test, for personal use
- Easy to use, with pre-filled sample tubes
- With special approval from the BfArM
HYGISUN Covid-19 rapid test for laypeople
The HYGISUN COVID-19 antigen rapid test (colloidal gold) is an antigen test approved by the BfArM in accordance with Section 11 (1) of the Medical Devices Act (MPG) for self-use by laypeople. The file number of the special approval is: 5640-S-058/21.
NASAL SWAB TEST
The HYGISUN COVID-19 antigen rapid test (colloidal gold) relies on the new test method in which the sample is simply taken from a saliva discharge, known colloquially as the “spit test”. This release of saliva enables a qualitative in vitro determination of the novel coronavirus antigen.
Related products
Sale!

 Multi Fold Hand Towel Dispenser White - Fits All Interfold Xpress Towels
Multi Fold Hand Towel Dispenser White - Fits All Interfold Xpress Towels  Centrefeed Blue Wiper Rolls 30 Roll Deal 5Pk - Kitchen Catering Wipe
Centrefeed Blue Wiper Rolls 30 Roll Deal 5Pk - Kitchen Catering Wipe  Henry Hoover Vacuum Cleaner Centre Selco.ie
Henry Hoover Vacuum Cleaner Centre Selco.ie  Brown Kraft Hotdog Box Food To Go, Takeaway Food Packaging
Brown Kraft Hotdog Box Food To Go, Takeaway Food Packaging  Back Pack Vacuum Cleaner -Battery Hoover -
Back Pack Vacuum Cleaner -Battery Hoover -  White Centrefeed Roll 2ply 700sh 6 Pack - Pure Soft Wiping Paper
White Centrefeed Roll 2ply 700sh 6 Pack - Pure Soft Wiping Paper  Amethyst Deodoriser For Odour Removal
Amethyst Deodoriser For Odour Removal  Biohazard Sharps Bin Box Large Medical Bin 4 Litre Needle Box
Biohazard Sharps Bin Box Large Medical Bin 4 Litre Needle Box  Microsafe Disinfectant Surface Wipes - Antibacterial Protection
Microsafe Disinfectant Surface Wipes - Antibacterial Protection